RECOMMENDATION FOR ADSORPTION PROCESS
Adsorption is an exothermic process and enthalpy change is always negative. Download high-res image 256KB Download.

We Provide A Wide Range Of Air Dryers For Different Application As Per Your Requirement And Your Source Of Air Our Expertise Wi Gas Dryer Dryer Gas Compressor
Adsorption is a spontaneous process at constant pressure and temperature thus Gibbs free energy is also decreased.

. What Adsorption Means in Chemistry. H ads 100 kJmol I chemical bond formation in the order of chemical bond strengths I leads to reaction products. High heat of adsorption in the range of 40-400 kJ mol-1.
Force of attraction are Van der Waals forces. The adsorption process is one of the effective methods for removal of dyes from the waste effluent. This chapter is focused on the adsorption of gases in high-capacity solid adsorbents such as active carbon 1 or zeolites2 These commercial adsorbents owe their enormous.
Therefore adsorption can be considered an equilibrium-diffusion reaction process. The isotherm so identified will be used later in the modeling of the adsorption process. Adsorption as a separation process is widely applied in the manufacturing economy and everyday life.
Several methods for the treatment of dye wastewater are including filtration technology chemical treatment oxidation sedimentation adsorption and ion exchange. Two types of adsorption. The resulting isosteric heat of adsorption at 285 K is equal to 36 kJmol 1 thus proving that ammonia is likely to be chemically adsorbed.
Thermodynamics of Adsorption ALAN L. When adsorbate molecules are adsorbed on the surface freedom of movement of molecules become restricted and this results in decrease in entropy. The column will be regenerated with steam until the heptane partial pressure is reduced to 336 mm Hg.
The positive value of enthalpy. MYERS 1 Introduction The attachment of molecules to the surface of a solid by adsorption is a broad subject. The experimental factors affecting the batch mode of adsorption of various metals and inorganic anions are discussed in this book.
The results reveal that the naproxen adsorption process slowly increased with an increase in temperature. Synthetic resins and water purification. For RDP the adsorption process followed the Freundlich adsorption isotherm model at 25 C while it was more fitted to the Langmuir isotherm model at 45 C and all models at 35 C.
Off-Gas Adsorption Model Capabilities and Recommendations 01 March 2016 iii. The elemental contaminants have been categorized into four major categories ie. We demonstrate a COF recommendation system to match COFs with adsorption tasks by training a low rank model of an incomplete COF--adsorption-property matrix.
Among these methods adsorption is currently considered to be very suitable for wastewater treatment because of its simplicity and cost effectiveness Yadanaparthi et al2009 Kwon et al 2010. Yes it can be because adsorption is a weak reaction and the reaction could be endothermic or exothermic. Low heat of adsorption usually in the range of 20-40 kJ mol-1.
Biological degradation occurring on the granules complements the adsorption process in removing dissolved organic material. A low rank model trained on the observed COF adsorption property values provides i predictions of the missing COF adsorption property values and ii a map of COFs wherein COFs with similar. Off-gas treatment is required to reduce emissions from aqueous fuel recycling.
11Software License Manager SLM Installation and Reference Guide. The key difference between adsorption and desorption is that adsorption refers to the process by which some solid holds molecules of a gas or liquid or solute as a thin film whereas desorption refers to the release of an adsorbed substance from a surface. Ad A Peer-Reviewed Open Access Forum on the Study of Adsorption and Desorption Phenomena.
I Low heat of adsorption released. Adsorption performance is strongly influenced by mass transfer of the species between the solution and the adsorbent surfaces and the adsorption reaction rate. The presented results.
Superheated steam at 2475 C and one atmosphere is available to regenerate the column. It usually takes place at low temperature and decreases with increasing temperature. Up to 8 cash back Adsorption is one of the method that is in use for remediation of contaminated water.
Adsorption is facilitated by the large surface areas on the carbon granules which are attributable to its highly porous structure. Dyes removal is effectively used physical and chemical methods but this method is. It takes place at high temperature.
The adsorption process was feasible endothermic and spontaneous in nature supported by different surface active sites notably silanol aluminol and hydroxyl groups. 1 air and steam do not interfere with the adsorption of heptane on carbon 2 heptane. H ads 30 to 60 kJmol I Theoryvan der Waals attractions I easily reversible 2Chemical adsorption chemisorption.
A process in which atoms or molecules move from a bulk phase that is solid liquid or gas onto a solid or liquid surface. Getting adsorbed on a bacteria as a bacteriophage is the first step in the viral life cycle. An example is purification by adsorption where impurities are filtered from liquids or gases by their adsorption onto the surface of a high-surface-area solid such as activated charcoal.
Forces of attraction are chemical bond forces. I High heat of adsorption released. Adsorption is commonly used technique for the removal of metal ions from various industrial.
Carbon in certain configurations also functions as a filter. Join Leading Researchers in the Field and Publish With Us. The gas adsorption process consists to reproduce the active component from a.
Evaluating the products of innovative gas adsorption research requires increased computational simulation capability to more effectively transition from. Adsorption chillers combine adsorbents with refrigerants and use heat to provide a cooling effect. Thus the concentration velocity with a Langmuir isotherm is an increasing function of concentration.
The corresponding thermodynamic values G H and T S are -504 922 and 1422 kJmol-1 respectively. We saw earlier that the slope of the Langmuir isotherm is monotonically decreasing. Hi Dr Komalkant Adlak.
A carbon adsorption column is available to remove the heptane. The feed quality ebook you can be collected in adsim aspen flare system for an account or sequestration process manual enables access. Therefore the wave in an adsorption process will generally be a shock and the wave in a desorption process will be a spreading wave.

Application Of Adsorption Process For Effective Removal Of Emerging Contaminants From Water And Wastewater Sciencedirect
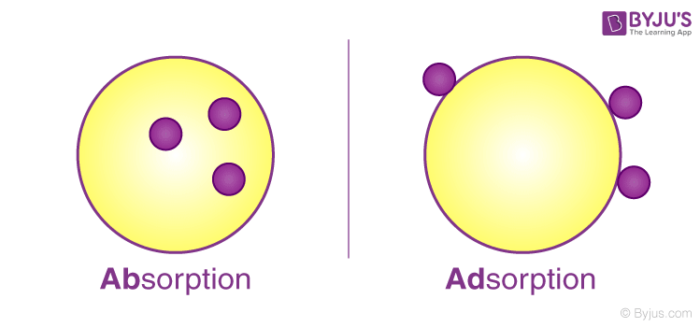
Factors Affecting The Extent Of Adsorption And Exothermic Nature

Civil Engineering Seminar Topics Activated Carbon Adsorption Activated Carbon Carbon Water Treatment

Adsorption Performance Of Modified Agricultural Waste Materials For Removal Of Emerging Micro Contaminant Bisphenol A A Comprehensive Review Sciencedirect

Guidelines For The Use And Interpretation Of Adsorption Isotherm Models A Review Sciencedirect

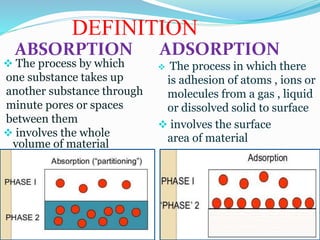

Belum ada Komentar untuk "RECOMMENDATION FOR ADSORPTION PROCESS"
Posting Komentar